댓글 쓰기 권한이 없습니다. 로그인 하시겠습니까?
Intrinsic size-dependent activity of Pt nanoparticles without masking by heterogeneous oxidation states of Pt for hydrolytic dehydrogenation of NH3BH3
| Journal | Int J. Energy Research |
|---|---|
| Author | Youngwon Ju,Taehoon Cho,Kichul Lee,Jiwoong Kim,Chang Won Yoon,Joohoon Kim |
| Citation | Int J Energy Res.2022;46:9771?9781 |
| DOI | https://doi.org/10.1002/er.7846 |
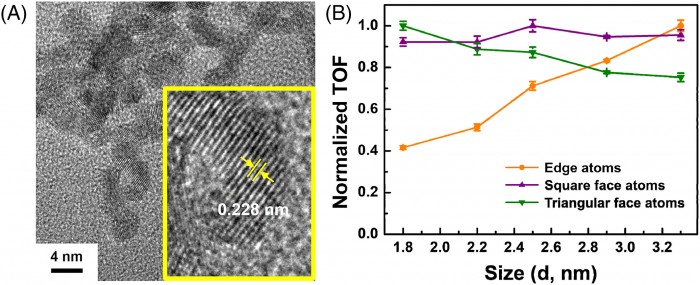
We report size-dependent activity of Pt nanoparticles for hydrolytic dehydrogenation of ammonia borane (AB, NH3BH3) in the size range of 1 to 4 nm without masking by heterogeneous oxidation states of Pt. Specifically, we synthesized five differently sized Pt nanoparticles encapsulated inside generation 6 poly(amidoamine) dendrimers with a uniform oxidation state of Pt in an effort to study the intrinsic size effect of Pt nanoparticles on their catalysis. The five differently sized Pt nanoparticles exhibited significant catalytic activity for hydrolytic dehydrogenation of AB under mild conditions. Interestingly, we found that the catalytic activity of the Pt nanoparticles encapsulated inside dendrimers was tunable even with the subnanometer changes in the sizes of Pt nanoparticles in the range of small sizes less than 4 nm. In particular, the Pt nanoparticle with a mean size of 2.5 nm exhibited the highest catalytic activity with a turnover frequency value of 242.3?±?7.1molH2 molPt?1 min?1 for hydrogen generation from the hydrolysis of AB at 25°C. The size-dependent activity of Pt nanoparticles was attributed to be geometric in nature, primarily due to the square face Pt atoms as the dominant active sites for the hydrolytic dehydrogenation of AB.
-
Read More

Three-Dimensional TEM Study of Dendrimer-Encapsulated Pt Nanoparticles for Visualizing Structural Characteristics of the Whole Organic-Inorganic Hybrid Nanostructure
Category2021 AuthorYoungwon Ju, Hyun Joo Ro, Yoon Sun Yi, Taehoon Cho, Seung Il Kim, Chang Won Yoon, Sangmi Jun, Joohoon Kim JournalThree-Dimensional TEM Study of Dendrimer-Encapsulated Pt Nanoparticles for Visualizing Structural Characteristics of the Whole Organic-Inorganic Hybrid Nanostructure CitationAnal. Chem. 2021, 93, 5, 2871?2878
-
Read More

Intrinsic size-dependent activity of Pt nanoparticles without masking by heterogeneous oxidation states of Pt for hydrolytic dehydrogenation of NH3BH3
Category2022 AuthorYoungwon Ju,Taehoon Cho,Kichul Lee,Jiwoong Kim,Chang Won Yoon,Joohoon Kim JournalInt J. Energy Research CitationInt J Energy Res.2022;46:9771?9781
-
Read More

Enhanced near-infrared electrochemiluminescence of Au nanoclusters treated with piperidine
Category2022 AuthorJae Hyun Kim, Jeongyun Choi, Jiwoo Kim, Joohoon Kim JournalBioelectrochemistry CitationBioelectrochemistry 147 (2022) 1
-
Read More
Efficient blue organic electrochemiluminescence luminophore based on a pyrenyl-phenanthroimidazole conjugate
Category2022 AuthorKwang-Myeong Kim, Jiwoo Kim, Joohoon Kim*, Jong-In Hong* JournalChemical Communications CitationChem. Commun., 2022, 58, 7542
-
Read More

Distinctive optical transitions of tunable multicolor carbon dots
Category2022 AuthorHyeong Seop Shim, Jun Myung Kim, Seonghyun Jeong, Youngwon Ju, Sung Jae Won, Jeongyun Choi, Sangwon Nam, Aniruddha Molla, Joohoon Kim* and Jae Kyu Song* JournalNanoscale Advances CitationNanoscale Adv., 2022,4, 1351-1358
Designed by sketchbooks.co.kr / sketchbook5 board skin
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
.png)


 Three-Dimensional TEM Study of Dendrimer-Encapsulate...
Three-Dimensional TEM Study of Dendrimer-Encapsulate...
 Enhanced near-infrared electrochemiluminescence of ...
Enhanced near-infrared electrochemiluminescence of ...