댓글 쓰기 권한이 없습니다. 로그인 하시겠습니까?
Galvanic Bipolar Electrode Arrays with Self-Driven Optical Readouts
| Journal | ACS Sensors |
|---|---|
| Author | Hyein Lee, Jiwoo Kim, Misol Hwang, and Joohoon Kim |
| Citation | ACS Sens. 2023, 8, 11, 4374?4383 |
| DOI | https://doi.org/10.1021/acssensors.3c01807 |

In this work, we report a bipolar electrode (BPE) array system with self-driven optical readouts of the faradic current flowing through the BPEs. The BPE array system is based on the spontaneous redox reactions that are respectively occurring at opposite poles of the BPEs with appropriate electrocatalysts on the poles; this system is analogous to one consisting of galvanic electrochemical cells. The galvanic BPE array system operates in a self-powered mode that requires there to be neither a direct electrical connection nor external electrical polarization to each BPE. Importantly, the appropriate electrocatalysts on the poles play a critical role in the galvanic BPE array system to induce the spontaneous redox reactions occurring at the poles of BPEs. Moreover, the galvanic BPE array system provides self-driven optical readouts, including fluorometric and colorimetric ones, to report the faradaic current resulting from the spontaneous redox reactions on the BPE poles. Based on the unique benefits that the galvanic BPE array system has over conventional BPEs, we demonstrated the promising potential of galvanic BPE arrays for the simple yet rapid and quantitative screening of electrocatalysts for the oxygen reduction reaction as well as sensitive sensing of H2O2 in parallel.
-
Read More
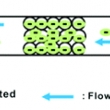
Hybridization of DNA to bead-immobilized probes confined within a microfluidic channel
Category-2009 AuthorKim, J.; Heo, J.; Crooks, R. M. JournalLangmuir CitationLangmuir, 2006, 22, 10130-10134
-
Read More
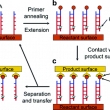
Transfer of surface polymerase reaction products to a secondary platform with conservation of spatial registration
Category-2009 AuthorKim, J.; Crooks, R. M. JournalJ. Am. Chem. Soc. CitationJ. Am. Chem. Soc., 2006, 128, 12076-12077
-
Read More
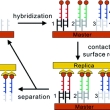
Replication of DNA microarrays from zip code masters
Category-2009 AuthorLin, H.; Kim, J.; Sun, L.; Crooks, R. M. JournalJ. Am. Chem. Soc. CitationJ. Am. Chem. Soc., 2006, 128, 3268-3272
-
Read More

Parallel fabrication of RNA microarrays by mechanical transfer from a DNA master
Category-2009 AuthorKim, J.; Crooks, R. M. JournalAnal. Chem. CitationAnal. Chem., 2007, 79, 8994-8999
-
Read More

Replication of DNA microarrays prepared by in situ oligonucleotide polymerization and mechanical transfer.
Category-2009 AuthorKim, J.; Crooks, R. M. JournalAnal. Chem. CitationAnal. Chem., 2007, 79, 7267-7274
Designed by sketchbooks.co.kr / sketchbook5 board skin
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
Sketchbook5, 스케치북5
.png)

